Description
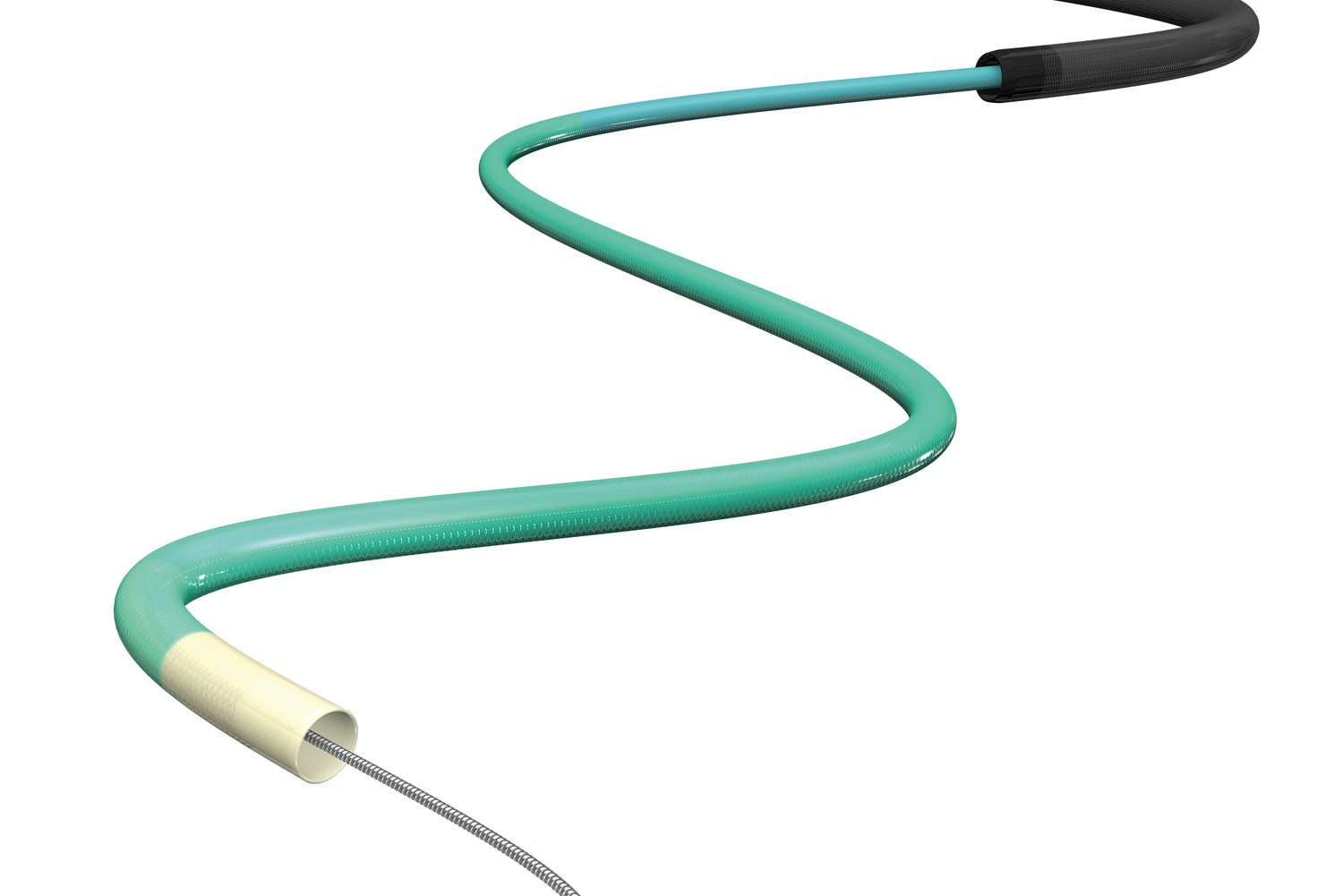
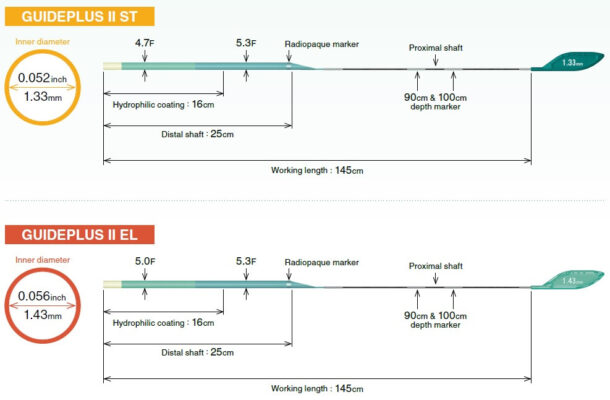
GUIDEPLUS™ II revolutionizes and simplifies the delivery of therapeutic devices in problematic cases while ensuring excellent crossability and access to the distal vasculature due to a well-balanced flexible shaft and hydrophilic coating.
- An atraumatic round tip comprising of flexible radiopaque material provides for safe delivery through the vasculature.
- The unique port shape allows the devices to be inserted safely and easily.
- 2 types available: Superior Trackability (ST) and Expanded Lumen (EL)
Frequently Asked Questions (FAQs)
1. What are the benefits of using a guide extension catheter?
Provides additional support for device delivery, improves access to difficult lesions, enhances the success rate of complex interventions, and reduces the risk of complications associated with inadequate support.
2. How does a guide extension catheter improve procedural outcomes?
By providing enhanced support and better device delivery pathways, it helps achieve successful stent placement and other interventions in challenging anatomical situations, leading to better patient outcomes.
3. How do you choose the appropriate guide extension catheter for a procedure?
The choice depends on factors such as the size and location of the lesion, vessel tortuosity, and the specific devices being delivered. Consultation with the interventional team and imaging assessments guide the selection.
GUIDEPLUS™ II revolutionizes and simplifies delivery of therapeutic devices in problematic cases while ensuring excellent crossability and access to the distal vasculature due to well balanced flexible shaft and hydrophilic coating.
4. When advancing GUIDEPLUS II, is the guiding catheter more likely to become disengaged compared to competitive products?
The flexibility of the shaft ensures superior vessel trackability and a reduction in resistance during insertion compared to competitive products. This results in allowing the device to be inserted more easily under reduced back-up force, does not rely on the guiding catheter positioning, and should result in the guiding catheter disengaging less than competitive product.
5. The shaft component is more flexible than competitive products, but how is the pushability of GUIDEPLUS II?
Due to the distal shaft being more flexible, the pushability is lower. Although GUIDEPLUS II has lower pushability, the highly hydrophilic coating and flexible shaft ensure that the catheter can be advanced with minimal resistance, even with lower back up.
6. Is there comparative data for pushability?
There is no comparative data on pushability. In a clinical setting, the required pushability is greater than the resistance required during insertion of the product. Given the resistance value incurred during insertion of the GUIDEPLUS II is lower than other products, no comparative data was attained in regards to pushability.
*The distal shaft of GUIDEPLUS II is comprised of 3 differing levels of stiffness, ensuring that the design enables push transmission from hub to tip.
7. When advancing GUIDEPLUS II, is the guiding catheter more likely to become disengaged compared to competitive products?
The flexibility of the shaft ensures superior vessel trackability and a reduction in resistance during insertion compared to competitive products. This results in allowing the device to be inserted more easily under reduced back-up force, does not rely on the guiding catheter positioning, and should result in the guiding catheter disengaging less than competitive product.
8. Can the GUIDEPLUS II still be advanced to the distal vasculature if it incurs kinking?
Should the proximal shaft incur kinking:
It is recommended to be used without excessive force.
Should the distal shaft incur kinking:
If kinking is determined when product is in-vivo, the product should not be advanced further, with it immediately removed and the condition of the catheter confirmed.
Given no visual deformity or stretching of the catheter, a device should be advanced (in-vitro) and if no resistance is incurred during device insertion, the catheter can be reinserted within the patient.
9. If GUIDEPLUS II incurs kinking is it still permissible to continue the procedure (undertaking stent delivery)?
Regarding distal shaft:
If kinking incurs in-vivo, the product should be retrieved and the condition confirmed (in-vitro). Should visual observation confirm product is ok and without any stretching, a device should be advanced (in-vitro) and given no resistance during insertion the catheter can be reinserted within the patient.
However, other than the above condition, a device used in combination may become trapped, and have potential to cause further complications, in which case it is not recommended to continue use.
If kinking occurs during delivery or after stenting, remove the entire system.
10. Can GUIDEPLUS II be firmly advanced without issue?
The distal shaft is relatively elastic and rapidly inserting the product will result in the transition of force to the shaft and not result in the catheter advancing distally.
If resistance is incurred during delivery, rather than overtly pushing the catheter, a delivery technique of advancing the catheter with slow vibrations is recommended.
11. Can GUIDEPLUS II be delivered beyond an implanted stent?
Care should be taken not to cause damage or stent deformation when inserting GUIDEPLUS II beyond a previously implanted stent. If the catheter incurs resistance within the stent, it should not be advanced further.
Delivery should not be a concern if the stent is fully apposed to the vessel wall, however there is potential for the tip to become caught on the stent struts and further insertion should be avoided if there is stent mal-apposition.
The catheter tip stiffness is far less than the strut of the stent, and given the tip comes into contact with stent struts, and is designed to ensure minimal stent deformation.
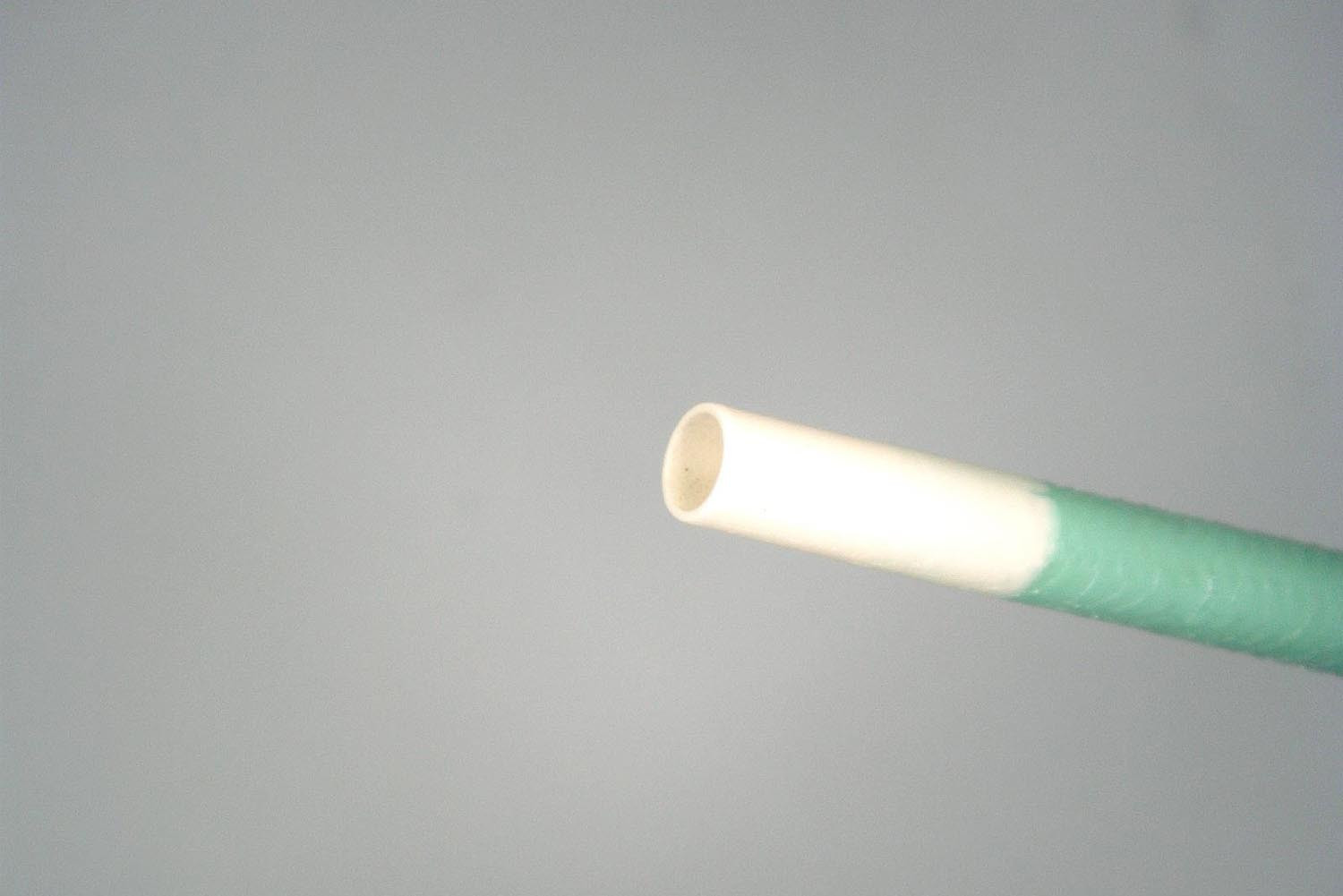
12. Where is the tip marker located?
It is located within the white resin marker portion (raqiopaque material is mixed with the resin).
13. Is it more likely to cause damage to the device compared to competitive products?
The answer depends on the device component
(a RX port, b proximal shaft, c distal shaft, d tip)
a The RX port is designed to be resistant to damage.
b The profile of proximal shaft is lower than competitors, and therefore has potential for kinking.
c The distal shaft is extremely flexible, and excessive force will result in a stretching of the shaft.
c The distal shaft is flexible, and is considered more difficult to kink compared to competitors.
14. Is it more likely to incur kinking compared to competitive products?
d The tip is flexible, and has greater shape memory retention compared to other products and is more difficult to incur collapsing of the tip.
15. Is there potential for GUIDEPLUS II to cause perforation or dissection?
Compared to other products, the shaft and tip is more flexible and reduces risk of complications.
There is risk of perforation or dissection should contrast media be introduced when in a wedged condition (as with any product of this nature).
16. Is there risk of aortic dissection?
This device is advanced within the guiding catheter. Therefore causing damage to the aorta is not a consideration.
17. Is there potential for complications when used in complex lesions?
There is no considered risk when used in complex lesions.
However, there is a report of a stent being inflated with the proximal portion of it being within the GUIDEPLUS II catheter and subsequent removal of the catheter encountered difficulty.
Further, there is a report of contrast injection being made via use of an auto-injector that led to the expansion of a dissection.
18. Is there potential for slow flow/no flow to occur when GUIDEPLUS II is delivered to the distal vasculature?
Unlike a balloon or stent that is used for dilatation of the vessel, GUIDEPLUS II has a low risk of causing emboli. Given the large outer diameter of the device, care should be taken when crossing through a lesion in order not to cause a distal emboli.
19. Is there potential to cause damage to devices used in combination with GUIDEPLUS II? Are there defective product reports relating to GUIDEPLUS II causing damage to devices used in combination?
There are reports of devices becoming trapped within the GUIDEPLUS II catheter.
These include:
During insertion and removal of a device, resistance suddenly increased, and during catheter retrieval the shaft was stretched and the device became trapped.
Due to the enlarged profile of devices post inflation, balloons and stent balloons have become trapped.
These situations can be avoided by simultaneously removing both GUIDEPLUS II and device used in combination, when resistance is incurred during device manipulation.